| Product: | Aurintricarboxylic acid | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Catalog Number: | 16290 | ||||||||
| CAS Number: | 4431-00-9 | ||||||||
| Synonyms: |
5,5’-(3-Carboxy-4-oxocyclohexa-2,5-dienylidenemethylene)di(salicylic acid); ATA |
||||||||
| Pricing: |
|
||||||||
| Formula: | C22H14O9 | ||||||||
| Chemical Purity: | >85% | ||||||||
| Molecular Weight: | 422.35 | ||||||||
| Structure: |
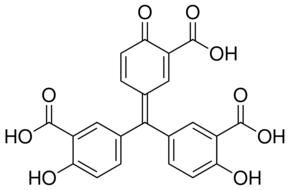
|
||||||||
| Appearance: | Solid | ||||||||
| Category: | Biochemicals and Reagents | ||||||||
| Stability: | Stable under recommended storage conditions. | ||||||||
| Storage: |
Keep container tightly closed in a dry and well ventilated place |
||||||||
| Transportation: |
Non-hazardous for transport |
||||||||
| Literature References: |
Bartoszewski, R.A., et al., A Synonymous Single Nucleotide Polymorphism In DeltaF508 CFTR Alters The Secondary Structure Of The MRNA And The Expression Of The Mutant Protein. J. Thorac. Cardiovasc. Surg. 285, 28741-8, (2010); Gonzalez, R.G., et al., Mechanism of action of polymeric aurintricarboxylic acid, a potent inhibitor of protein--nucleic acid interactions. Biochemistry 19, 4299-4303, (1980); Benchokroun, Y., et al., Aurintricarboxylic acid, a putative inhibitor of apoptosis, is a potent inhibitor of DNA topoisomerase II in vitro and in Chinese hamster fibrosarcoma cells. Biochem. Pharmacol. 49, 305-313, (1995); Rui, H., et al., Activation of the Jak2-Stat5 signaling pathway in Nb2 lymphoma cells by an anti-apoptotic agent, aurintricarboxylic acid. J. Biol. Chem. 273, 28-32, (1998); Okada, N., and Koizumi, S., Tyrosine phosphorylation of ErbB4 is stimulated by aurintricarboxylic acid in human neuroblastoma SH-SY5Y cells. Biochem. Biophys. Res. Commun. 230, 266-269, (1997); Okada, N., and Koizumi, S., A neuroprotective compound, aurintricarboxylic acid, stimulates the tyrosine phosphorylation cascade in PC12 cells. J. Biol. Chem. 270, 16464, (1995); Aronica, E.M., et al., Aurintricarboxylic acid prevents GLUR2 mRNA down-regulation and delayed neurodegeneration in hippocampal CA neurons of gerbil after global ischemia. Proc. Natl. Acad. Sci. U. S. A. 95, 7115-7120, (1998); Posner, A., et al., Aurintricarboxylic acid is an inhibitor of mu- and m-calpain. Biochem. Mol. Biol. Int. 36, 291-299, (1995); Beil. 10,1050; FT-IR 2 (3), 4127:D / FT-IR 1 (2), 237:A / IR-Spectra (3), 984:H / IR-Spectra (2), 863:D / RegBook 1 (2), 2775:C / Sigma FT-IR 1 (2), 100:D / Stains and Dyes Ref, 105 / Structure Index 1, 440:C:8 |
||||||||
| MSDS: | |||||||||
| Description: |
Readily polymerizes in aqueous solution, forming a stable free radical that inhibits protein-nucleic acid interactions. It is a potent inhibitor of ribonuclease and topoisomerase II by preventing the binding of the nucleic acid to the enzyme. It stimulates tyrosine phosphorylation processes including the Jak2/STAT5 pathway in NB2 lymphoma cells, ErbB4 in neuroblastoma cells, and MAP kinases, Shc proteins, phosphatidylinositide 3-kinase and phospholipase Cγ in PC12 cells. Inhibits apoptosis. It prevents down-regulation of Ca2+ -impermeable GluR2 receptors and inhibits calpain, a Ca2+ -activated protease that is activated during apoptosis. |
