| Product: | Chloranilic acid | ||||
|---|---|---|---|---|---|
| Catalog Number: | 16471 | ||||
| CAS Number: | 87-88-7 | ||||
| Synonyms: | 2,5-Dichloro-3,6-dihydroxy-p-benzoquinone | ||||
| Pricing: |
|
||||
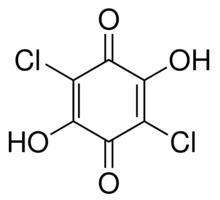
| Formula: | C6H2Cl2O4 | ||||
| Molecular Weight: | 208.98 | ||||
| Structure: |
|
||||
| Appearance: | Red powder | ||||
| Category: | Biochemicals and Reagents | ||||
| Stability: | Stable under recommended storage conditions. | ||||
| Storage: |
Keep container tightly closed in a dry and well ventilated place. |
||||
| Transportation: |
Non-hazardous for transport |
||||
| Literature References: |
M. Chang, et al., Dimensionality Control of Vapochromic Hydrogen-Bonded Proton-Transfer Assemblies Composed of a Bis(hydrazone)iron(II) Complex Inorg. Chem. 50, 8308-8317, (2011); G. Bator, et al., 4,4'-, 5,5'-, and 6,6'-dimethyl-2,2'-bipyridyls: The structures, phase transitions, vibrations, and methyl group tunneling of their complexes with chloranilic acid J. Chem. Phys. 135, 044509/1-044509/11, (2011); P. Rajakannu, et al., Adaptation toward Restricted Conformational Dynamics: From the Series of Neutral Molecular Rotors Organometallics 30, 3168-3176, (2011); L. Yu, et al, Spectrophotometric determination of metoprolol tartrate based on the charge transfer reaction with chloranilic acid Huaxue Shiji 32, 1003-1005, (2010); K. Molcanov and B. Kojic-Prodic, Salts and co-crystals of chloranilic acid with organic bases: is it possible to predict a salt formation? CrystEngComm 12, 925-939, (2010); N. P. E. Barry, et al., Anticancer activity of osmium metalla-rectangles Dalton Trans. 39, 2816-2820, (2010); Merck 14,2079; Beil. 8,IV,2707; FT-IR 2 (1), 716:D / FT-IR 1 (1), 455:B / IR-Spectra (2), 240:G / IR-Spectra (3), 269:E / RegBook 1 (1), 493:L / Sigma FT-IR 1 (2), 369:A / Structure Index 1, 71:E:2 |
||||
| MSDS: | |||||
| Applications: |
Reactant involved in: |
