| Product: | Camptothecin | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalog Number: | 17399 | ||||||||||||
| CAS Number: | 7689-03-4 | ||||||||||||
| Synonyms: | (S)-(+)-Camptothecin | ||||||||||||
| Pricing: |
|
||||||||||||
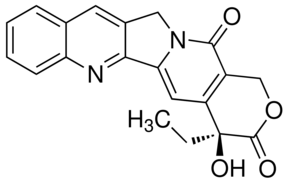
| Formula: | C20H16N2O4 | ||||||||||||
| Chemical Purity: | ~95% | ||||||||||||
| Molecular Weight: | 348.35 | ||||||||||||
| Structure: |
|
||||||||||||
| Appearance: | Powder | ||||||||||||
| Category: | Anti-cancer compounds | ||||||||||||
| Stability: | Stable under recommended storage conditions | ||||||||||||
| Storage: |
Keep container tightly closed in a dry and well-ventilated place. Recommended storage temperature: 2-8°C |
||||||||||||
| Transportation: |
IATA: Hazard Class: 6.1; UN Number: UN1544; Packing Group: III; Shipping Name: Alkaloids, solid, n.o.s ((S)-(+)-Camptothecin) |
||||||||||||
| Literature References: |
Fan, Y., et al., Molecular modeling studies of the DNA-topoisomerase I ternary cleavable complex with camptothecin. J. Med. Chem. 41, 2216-2226, (1998); Pommier, Y., et al., Mechanism of action of eukaryotic DNA topoisomerase I and drugs targeted to the enzyme. Biochim. Biophys. Acta 1400, 83-105, (1998); Desai, S. D., et al., Ubiquitin-dependent destruction of topoisomerase I is stimulated by the antitumor drug camptothecin. J. Biol. Chem. 272, 24159-24164, (1997); Beidler, D.R., and Cheng, Y.C., Camptothecin induction of a time- and concentration-dependent decrease of topoisomerase I and its implication in camptothecin activity. Mol. Pharmacol. 47, 907-914, (1994); Borovitskaya, A.E., and D-Arpa, P., Replication-dependent and -independent camptothecin cytotoxicity of seven human colon tumor cell lines. Oncol. Res. 10, 271-276, (1998); Morris. E.J., and Geller, H.M., Induction of neuronal apoptosis by camptothecin, an inhibitor of DNA topoisomerase-I: evidence for cell cycle-independent toxicity. J. Cell Biol. 134, 757-770, (1996); Kaufmann, S. H., Cell death induced by topoisomerase-targeted drugs: more questions than answers. Biochim. Biophys. Acta 1400, 195-211, (1998); Chung, J.W., et al., Pseudomonas Aeruginosa Eliminates Natural Killer Cells Via Phagocytosis-induced Apoptosis. PLoS Pathog. 5, e1000561, (2009); Simpson, N.E., et al., High Levels Of Hsp90 Cochaperone P23 Promote Tumor Progression And Poor Prognosis In Breast Cancer By Increasing Lymph Node Metastases And Drug Resistance. Cancer Res. 70, 8446-56, (2010); Uday Bhanu, M., and Kondapi, A.K., Neurotoxic Activity Of A Topoisomerase-I Inhibitor, Camptothecin, In Cultured Cerebellar Granule Neurons. Neurotoxicology 31, 730-7, (2010); Lienkamp, S., et al., Inversin Relays Frizzled-8 Signals To Promote Proximal Pronephros Development. Proc. Natl. Acad. Sci. U. S. A. 107, 20388-93, (2010); Merck 14,1735; FT-IR 2 (3), 4267:D / Structure Index 1, 462:C:1 |
||||||||||||
| MSDS: | |||||||||||||
| Description: |
(S)-(+)-Camptothecin binds irreversibly to the DNA-topoisomerase I complex, inhibiting the reassociation of DNA after cleavage by topoisomerase I and traps the enzyme in a covalent linkage with DNA. The enzyme complex is ubiquinated and destroyed by the 26S proteasome, thus depleting cellular topoisomerase I. Blocks the cell cycle in S-phase at low does and induces apoptosis in a large number of normal and tumor cell lines by cell cycle-dependent and cell cycle-independent processes. |
