| Product: | Cyclophosphamide Monohydrate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Catalog Number: | 17429 | ||||||||
| CAS Number: | 6055-19-2 | ||||||||
| Synonyms: |
Cyclophosphamide monohydrate; Cytotoxan; 2-[Bis(2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide |
||||||||
| Pricing: |
|
||||||||
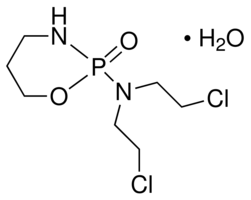
| Formula: | C7H15Cl2N2O2P • H2O | ||||||||
| Chemical Purity: | >97% | ||||||||
| Molecular Weight: | 279.10 | ||||||||
| Structure: |
|
||||||||
| Appearance: | White crystalline | ||||||||
| Category: | Anti-cancer compounds | ||||||||
| Stability: | Stable under recommended storage conditions. | ||||||||
| Storage: |
Keep container tightly closed in a dry and well-ventilated place. Recommended storage temperature: 2-8°C. |
||||||||
| Transportation: |
IATA: Hazard Class: 6.1; UN Number: UN3464; Packing Group: III; Shipping Name: Organophosphorus compound, toxic, solid, n.o.s. (Cyclophosphamide monohydrate) |
||||||||
| Literature References: |
Cullion, K., et al., Targeting The Notch1 And MTOR Pathways In A Mouse T-ALL Model. Blood 113, 6172-81, (2009); Tammam, J., et al., Down-regulation Of The Notch Pathway Mediated By A Gamma-secretase Inhibitor Induces Anti-tumour Effects In Mouse Models Of T-cell Leukaemia. Br. J. Pharmacol. 158, 1183-95, (2009); Guerriero, J.L., et al., DNA Alkylating Therapy Induces Tumor Regression Through An HMGB1-Mediated Activation Of Innate Immunity. J. Immunol. 186, 3517-26, (2011); Gibson, L.F., et al., Regulation of BAX and BCL-2 expression in breast cancer cells by chemotherapy. Breast Cancer Res. Treat. 55, 107-117, (1999); Ren, S., et al., Inhibition of human aldehyde dehydrogenase 1 by the 4-hydroxycyclophosphamide degradation product acrolein. Drug Metab. Dispos. 27, 133-137, (1999); Inagawa, H., et al., Mechanisms by which chemotherapeutic agents augment the antitumor effects of tumor necrosis factor: involvement of the pattern shift of cytokines from Th2 to Th1 in tumor lesions. Anticancer Res. 18, 3957-3964, (1998); Merck 14,2747; Aldrich MSDS 1, 537:C / Corp MSDS 1 (1), 992:C / FT-IR 1 (1), 920:C / FT-IR 2 (1), 1582:D / FT-NMR 1 (1), 1500:B / IR-Spectra (3), 552:G / NMR-Reference 2 (2), 872:B / RegBook 1 (1), 1119:E / Sax 6, 1277 / Sigma FT-IR 1 (2), 418:D / Structure Index 1, 173:D:4 |
||||||||
| MSDS: | |||||||||
| Description: |
Cyclophosphamide is a cytotoxic nitrogen mustard derivative widely used in cancer chemotherapy. It cross-links DNA, causes strand breakage, and induces mutations. Its clinical activity is associated with a decrease in aldehyde dehydrogenase 1 (ALDH1) activity. |
