| Product: | 5-Azacytidine | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Catalog Number: | 17315 | ||||||||
| CAS Number: | 320-67-2 | ||||||||
| Synonyms: |
Ladakamycin; 4-Amino-1-(β-D-ribofuranosyl)-1,3,5-triazin-2(1H)-one |
||||||||
| Pricing: |
|
||||||||
| Formula: | C8H12N4O5 | ||||||||
| Chemical Purity: | >98% | ||||||||
| Molecular Weight: | 244.20 | ||||||||
| Structure: |
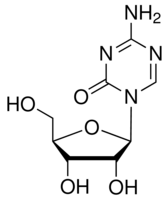
|
||||||||
| Appearance: | White powder | ||||||||
| Category: | Anti-cancer compounds | ||||||||
| Stability: | Stable under recommended storage conditions. | ||||||||
| Storage: |
Keep container tightly closed in a dry and well-ventilated place. |
||||||||
| Transportation: |
Non-hazardous for transport |
||||||||
| Literature References: |
Makino, H., et al., Mesenchymal To Embryonic Incomplete Transition Of Human Cells By Chimeric OCT4/3 (POU5F1) With Physiological Co-activator EWS. Exp. Cell Res. 315, 2727-40, (2009); Long, J., et al., Multiple Distinct Molecular Mechanisms Influence Sensitivity And Resistance To MDM2 Inhibitors In Adult Acute Myelogenous Leukemia. Blood 116, 71-80, (2010); Kuo, C.C., et al., Selective Activation Of SHP2 Activity By Cisplatin Revealed By A Novel Chemical Probe-based Assay. Biochem. Biophys. Res. Commun. 391, 230-4, (2010); Chang, J., et al., Nicotinamide Adenine Dinucleotide (NAD)-regulated DNA Methylation Alters CCCTC-binding Factor (CTCF)/cohesin Binding And Transcription At The BDNF Locus. Proc. Natl. Acad. Sci. U. S. A. 107, 21836-41, (2010); Kusaba H., et al., Association of 5′-CpG demethylation and altered chromatin structure in the promoter region with transcriptional activation of the multidrug resistance 1 gene in human cancer cells. Eur. J. Biochem. 262, 924-932, (1999); Broday L., et al., 5-Azacytidine induces transgene silencing by DNA methylation in Chinese hamster cells. Mol. Cell. Biol. 19, 3198-3204, (1999); Qian X., et al., DNA methylation regulates p27kip1 expression in rodent pituitary cell lines. Am. J. Pathol. 153, 1475-1482, (1998); Canova C., et al., Epigenetic control of programmed cell death: inhibition by 5-azacytidine of 1,25-dihydroxyvitamin D3-induced programmed cell death in C6.9 glioma cells. Mech. Ageing Dev. 101, 153-166, (1998); Merck 14,890; Aldrich MSDS 1, 139:B / Corp MSDS 1 (1), 321:D / FT-IR 2 (3), 3896:C / FT-IR 1 (2), 831:D / FT-NMR 1 (3), 418:B / IR-Spectra (2), 1191:F / IR-Spectra (3), 1367:E / RegBook 1 (2), 2603:A / Sax 6, 332 / Sigma FT-IR 1 (1), 758:C / Structure Index 1, 411:B:5 |
||||||||
| MSDS: | |||||||||
| Description: |
A potent growth inhibitor and cytotoxic agent; inhibits DNA methyltransferase, an important regulatory mechanism of gene expression, gene activation and silencing. Causes DNA demethylation or hemi-demethylation, creating openings that allow transcription factors to bind to DNA and reactivate tumor suppressor genes |
