Patents
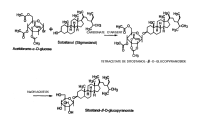
READILY ABSORBABLE PHYTOSTEROL GLYCOSIDES TO TREAT HYPERCHOLESTEROLEMIAFull text:WO9918977 Submitted: April 22, 1999 MEDICAL ISOTOPES INC [US] (WO1999018977) READILY ABSORBABLE PHYTOSTEROL GLYCOSIDES TO TREAT HYPERCHOLESTEROLEMIA
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
READILY ABSORBABLE PHYTOSTEROLS TO TREAT HYPERCHOLESTERROLEMIAFull text:WO9939715 Submitted: August 12, 1999 STOHLER ERIC [US],MEDICAL ISOTOPES INC [US] 1. (WO1999039715) READILY ABSORBABLE PHYTOSTEROLS TO TREAT HYPERCHOLESTERROLEMIA
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PHYTOSTEROL FORMULATIONS TO LOWER CHOLESTEROL ABSORPTIONFull text:WO9953925 Submitted: October 28, 1999 STOHLER ERIC [US],MEDICAL ISOTOPES INC [US] 1. (WO1999053925) PHYTOSTEROL FORMULATIONS TO LOWER CHOLESTEROL ABSORPTION
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||